Today, from Revio, we would like to share with you the latest updates on the scientific recommendations that European Medicines Agency’s Committee for Advanced Therapies (CAT) delivers on whether a potential new medicine can be classified as an advanced therapy medicinal product (ATMP). Don´t miss their Recommendations on Classification of ATMPs!
We have talked before about the CAT’s procedure on ATMP Classification before, you can check it on our post “Update on EMA’s procedural advice on classification of ATMPs”.
The CAT makes public the list of medicines assessed and recommended classifying as ATMPs since March 2019. This list is updated on a quarterly basis, they made the last update on May 2022. The document is a summary for public release of the classification of ATMPs, where they delete commercially confidential information from the original text.
The information contained in this list relates to the active substance, public indication, classification conclusions, outcome, and date of the CAT recommendations. Check the summary of the latest scientific recommendations in the following table:
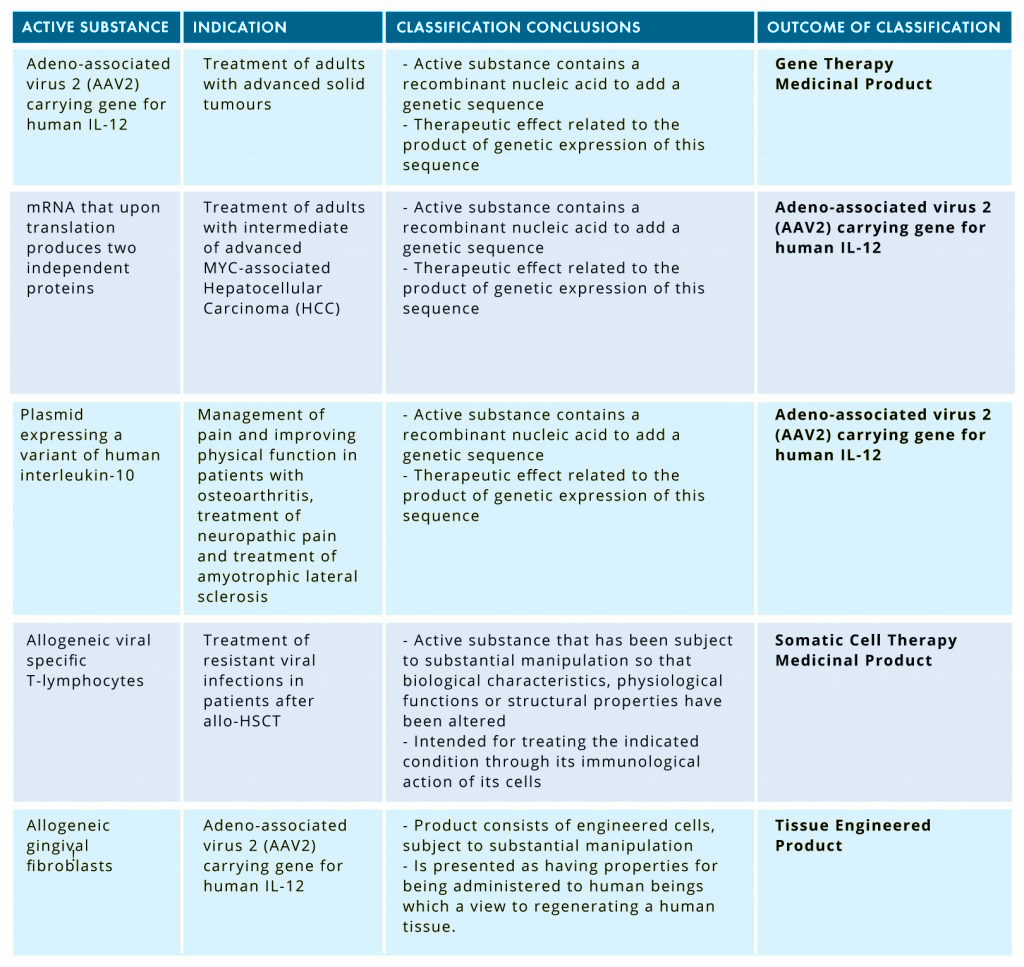
This public list contains now up to 76 scientific recommendations on different active substances and the CAT opinion on whether they are or not classified as advanced therapies. It may be of help to sponsors who are developing new cell- or gene-based medicines that could be classified as ATMPs. You can download the current list here!
You can also check the official website to consult the archive of every ATMP Classification procedure from June 2009 to March 2019 in this link
Nevertheless, we have launched a dedicated webpage to bring you the latest updates, guidance and developments. You can also follow us on LinkedIn.
We hope you find this useful and of interest. If you would like to discuss any of these updates with the team at Revio, please get in touch here.