The European Medicines Agency (EMA) publishes every year an annual report providing an overview of EMA’s work together with the European medicines regulatory network. In these annual reports you can find:
- Key achievement in protecting and promoting public and animal health in the EU.
- Reflections by EMA staff and its partners and stakeholders on topics of major interest in medicine and health.
- Key figures, including core statistics that highlight the main outcomes of the Agency’s activities and interesting trends and changes observed in recent years.
2022 Annual Report
- Health emergencies
Emerging public health emergencies remained a key focus area for EMA in 2022. Furthermore, new vaccines and treatment options were added to the EU’s arsenal against COVID-19. Additionally, when an outbreak of the monkeypox virus presented an additional challenge to public health, the established crisis tools were utilized. This ensured a coordinated EU response and the recommendation of various vaccines and treatments.
- Human medicines
In 2022, EMA recommended 89 human medicines for marketing authorisation, 41 of which had a new active substance. Moreover, a representation of medicines approved in 2022 that signifies significant progress in their therapeutic areas is displayed in the next figure from the report. Additionally, many of these progressive medicines are classified as advanced therapy medicinal products
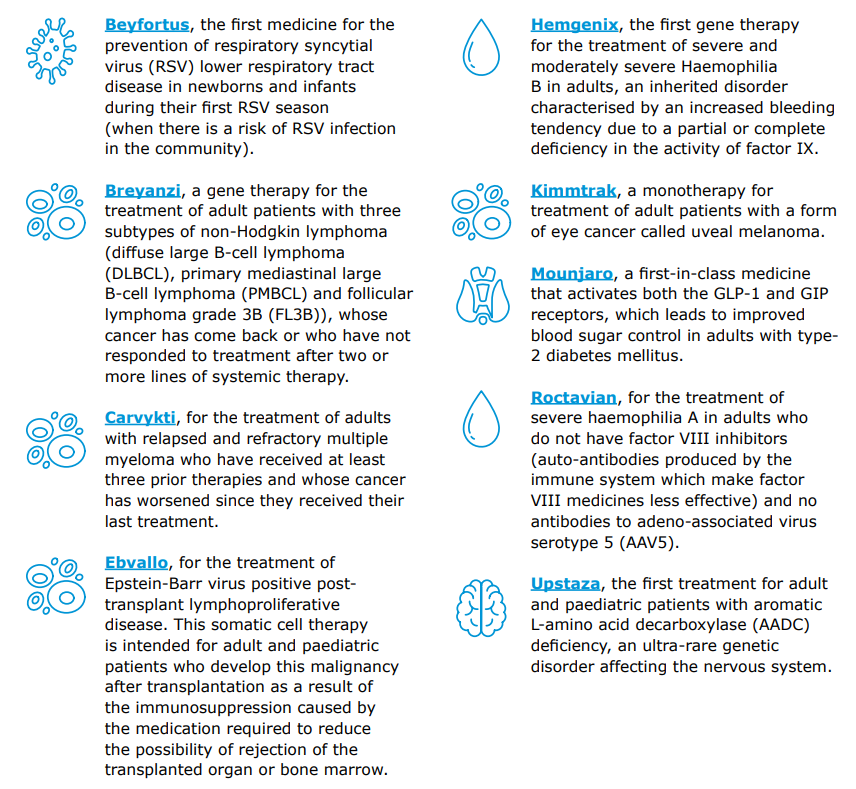
EMA 2022 annual report. Selection of medicines approved in 2022 that represent significant progress in their therapeutic areas.
- Clinical Trials
On January 2022 the Clinical Trials Regulation (CTR) entered into the application, harmonizing the submission, assessment and supervision processes for clinical trial based on the Clinical Trials Information System (CTIS).
This system serves as a single-entry point for sponsors and regulators of clinical trials for the submission and assessment of clinical trial data, including a public database for healthcare professions, patients and the public.
Although, in 2022, CTIS remained voluntary (which changed on 31 January 2023 for initial clinical trial applications), over 200 initial clinical trial application was authorised, and more than 200 were under the evaluation with the new system at the end of the year.
- Advanced Therapies
Advanced therapy medicinal products (ATMPs) are medicines based on genes or cells with potential for groundbreaking new treatment. Particularly important for severe, untreatable, or chronic diseases for which conventional approaches are inadequate.
- The Committee for Advanced Therapies (CAT) is responsible for assessing these medicines, and it prepares a draft opinion on each ATMP application before the CHMP adopts the final opinion. Also, the CAT provides scientific recommendations on the classification of a medicine as an ATMP.
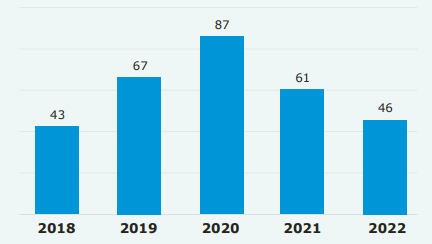
EMA 2022 annual report. Adopted recommendations of advanced therapy classifications.
In 2022, the CAT received 51 requests for ATMP classification and adopted 46 recommendations. For marketing authorisation, six ATMPs were recommended by the CHMP, compared to two in 2021. This confirms a rising trend in the number of application and approvals of advanced therapies in the EU.
The six ATMPs approved in 2022 were:
- Breyanzi
- Carvykti
- Ebvallo
- Hemgenix
- Roctavian
- Upstaza
If you want to know more about these medicines, we covered them all in our review on EMA’s 2022 Highlights on Advanced Therapies. You can check the publication here! (https://reviopharma.com/emas-2022-highlights-on-human-medicines-and-advanced-therapies)
This is some of the information that you can find in the new EMA’s 2022 annual report, if you want to know more about it you can check the public official document here! https://www.ema.europa.eu/en/documents/annual-report/2022-annual-report-european-medicines-agency_en.pdf
Nevertheless, we have launched a dedicated webpage to bring you the latest updates, guidance and developments. You can also follow us on LinkedIn.We hope you find this useful and of interest. If you would like to discuss any of these updates with the team at REVIO, please get in touch here.




