Today, from Revio, we would like to share with you a summary of the Food and Drug Administration (FDA) draft Guideline on Studying Multiple Versions of a Cellular or Gene Therapy Product in an Early-Phase Clinical Trial.
In this guidance, FDA provides recommendations for studies that evaluate multiple versions of a cellular or gene therapy product. It includes how to organize and structure the Investigational New Drug (IND), submit new information and report adverse events.
An ‘’umbrella’’ trial, a trial that study multiple products in parallel for a particular disease or condition under a master protocol, allows more efficient product development. This guidance focuses on a certain type of umbrella trial, where the products are multiple versions of a cellular or gene therapy product for a single disease. For example, a sponsor investigating an autologous chimeric antigen receptor (CAR) T cell product may wish to also investigate a different version of the product, like an altered CAR protein domain to increase CAR activity, or a new cell source such as an allogeneic donor. In this case, the different product versions are considered individual investigational drugs, but they could be simultaneously evaluated within the same overall trial.
SUBMISSION OF INFORMATION TO INDS
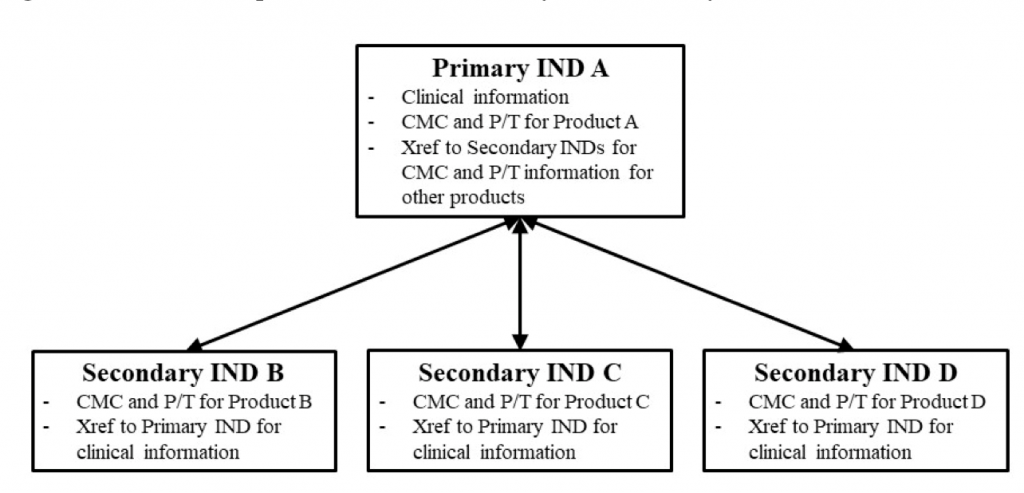
In this framework, INDs will be referred as ‘’Primary’’ or ‘’Secondary’’. Distinguishing which IND will include clinical information about the trial (Primary IND) and which INDs will not include clinical information and will refer to the Primary IND (Secondary INDs).
Clinical Study with two different versions of the investigational product (Product A and Product B):
FDA recommends that the sponsor submits two separate INDs for Product A and Product B. One of the INDs (IND A) will be considered the ‘’Primary’’ IND and should include Chemistry Manufacturing and Controls (CMC) and Pharmacology/Toxicology (P/T) information for Product A. IND B will be considered ‘’Secondary’’ IND, and will include CMC and P/T information for Product B.
Primary IND should cross-reference the Secondary IND for the CMC and P/T information. Secondary INDs should cross-reference the Primary IND for clinical information.
Adding Arms to the Study of the Cellular or Gene Therapy Product
If the arm to be added includes a new version of the investigational product (e.g., Product C) it is recommended that the sponsor submit:
- IND C with CMC and P/T information for Product C. Stating in the cover letter that it is a Secondary INF and specifying the Primary IND number.
- Amendment to IND A with the updated clinical protocol, including an arm for Product C. And including a cross-reference to the Secondary IND C for CMC and P/T information.
If the arm to be added does not include a new version of the investigational product (e.g., a new arm that will study Product B in combination with a marketed product, or a new arm that will study Product A and B together), then it is recommended to submit:
- An amendment to the Primary IND with the updated clinical protocol
- Any additional P/T information supporting the new arm to the relevant INDs.
Clinical Holds
In the event FDA issues an order placing the entire study on clinical hold, then all Primary and Secondary INDs will be placed on hold. If only one arm it’s placed on hold, then the Primary IND will be placed on partial hold and the relevant Secondary INDs will be placed on hold.
A response to each IND that was placed on hold will have to be summited.
Reporting
The sponsor must submit safety reports for an investigational product to all of the sponsor’s INDs that are relevant to that product.
Completion of Study or Arm(s)
If the sponsors would like to discontinue studying Product A (Primary IND), FDA does not recommend that the sponsor withdraw Primary IND A because it contains the relevant clinical information. It is recommended to submit a updated protocol to Primary IND A that no longer includes the arm with Product A.
Schematic Representation of the Primary and Secondary IND
This is some of the information you need to know if you are interested in the new draft guideline on Multiple Versions of a Cellular or Gene Therapy Product in an Ealy-Phase Clinical Trial. If you want to know more, you can check out the official guideline here.
We hope you find this useful and of interest. If you would like to discuss any of these updates with the team at REVIO, please get in touch here.
Also, we have a dedicated webpage to bring you the latest updates, guidance, and development. You can also follow us on LinkedIn.



