In the development of an Advanced Therapy Medicinal Product (ATMP) involving the donation of human tissues or cells to be used as starting materials, the control of the materials, traceability, and meeting the technical requirements and standards remains a big challenge in Chemistry, Manufacturing and Controls (CMC).
Today, from Revio, we present the legal framework you should follow in this aspect. In addition, we summarize the principal requirements that sponsors, and laboratories must pursue to ensure a good CMC assessment.
Legal Basis and Regulation
If tissues and cells are being used as starting materials in a medicinal product, applicants should consult:
- Directive 2004/23/EC also known as the European Tissues and Cells Directive, covering standards for donation, procurement and testing, processing, preservation, storage and distribution of human tissues and cells, as well as its technical implementing directives.
- Directive 2006/17/EC, the First Technical Directive, covering certain technical requirements for the donation, procurement and testing of human tissues and cells;
- Directive 2006/86/EC, the Second Technical Directive, covering standards for traceability, notification of serious adverse reactions and events, and requirements for coding processing, preservation, storage and distribution of human tissues and cells.
- Directive 2015/565, amending Directive 2006/86/EC, as regards certain technical requirements for the coding of human tissues and cells.
- Directive 2015/566, as regards the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells.
The European Tissues and Cell Directive does not apply to:
- Tissues and cells used as an autologous graft within the same surgical procedure.
- Blood and blood components.
- Organs or parts of organs if it is their function to be used for the same purpose as the entire organ in the human body.
Selection Criteria for Donors of human tissues and cells in medicinal products
Selection criteria for donors are based on an analysis of the risks related to the application of the specific cells/tissues. Indication of these risks must be identified by physical examination, review of the medical and behavioral history, biological testing, post-mortem examination (for deceased donors) and any other appropriate investigation.
There are some specific criteria to exclude donors from donation, such as:
Deceased donors:
Unknown cause of death, history of a disease of unknown etiology, presence of malignant disease, risk of transmission of diseases caused by prions, systemic infection at the time of donation.
Living donors:
History of a disease of unknown etiology, systemic infection, malignant disease, and in a case-by-case basis: pregnancy, breastfeeding, transmission of inherited conditions (in haematopoietic progenitor cells).
Laboratory Test Required for human tissues and cells in medicinal products
The following biological tests must be performed for all donors of tissues or cells as a minimum requirement:
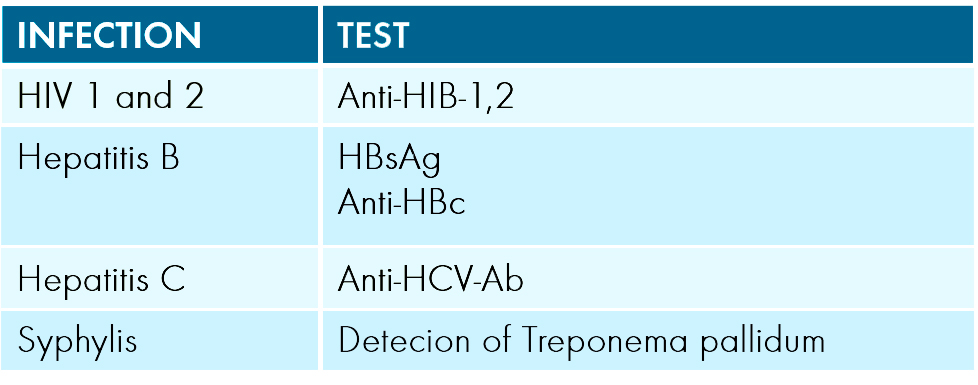
And other tests are required if certain conditions are met such as:
- HTLV-I for donors living in high-incidence areas.
- If anti-HBc is positive and HBsAg is negative, further investigations with a risk assessment to determine eligibility for clinical will be needed.
- Validated testing algorithm must be applied to exclude the presence of active infection with Treponema pallidum.
- Additional testing depending on the donor’s history and the characteristics of the cells and tissue to be donated (RhD, HLA, malaria, CMV, toxoplasma, EBV…).
Traceability
Known as the ability to locate and identify the tissue/cell during any step from procurement, through processing, testing and storage, to distribution to the recipient. And also, to locate and identify all relevant data relating to products and materials coming into contact with those tissues/cells.
Tissue establishments shall have effective and accurate system to uniquely identify, and label cells/tissues received and distributed. Also, they shall retain this data for at least 30 years, in an appropriate medium.
The appropriate accreditation, designation, authorisation or licensing system for tissue establishment and preparation processes is established in the different Member States.
We hope you find this summary on the donor requirements and traceability for tissues and cells in medicinal products useful and of interest. If you would like to discuss any of these updates with the team at REVIO, please get in touch here.
Also, we have a dedicated webpage to bring you the latest updates, guidance, and development. You can also follow us on LinkedIn.